In the world of life sciences, biological samples such as human tissues, blood, and other lab specimens play a crucial role in research, diagnostics, and therapeutic development. Transporting these sensitive materials safely and compliantly is vital to maintaining sample integrity, protecting patient safety, and ensuring regulatory adherence. Whether samples are destined for biobanks, laboratories, or clinical trial sites, secure and temperature-controlled logistics is essential.
In this blog, we’ll explore best practices to guarantee secure and compliant biological sample transport, helping organizations mitigate risks and optimize their supply chains.
Why Secure and Compliant Transport Matters
Biological samples are inherently vulnerable. Exposure to temperature fluctuations, contamination, or mishandling during transport can compromise sample quality, leading to invalid test results or failed research. Additionally, these materials often fall under strict regulatory frameworks such as Good Distribution Practice (GDP), International Air Transport Association (IATA) guidelines, and country-specific biosafety laws.
A robust transport strategy not only preserves sample viability but also ensures full compliance with evolving regulations—minimizing the risk of shipment delays, legal penalties, and reputational damage.
Best Practices for Biological Sample Transport
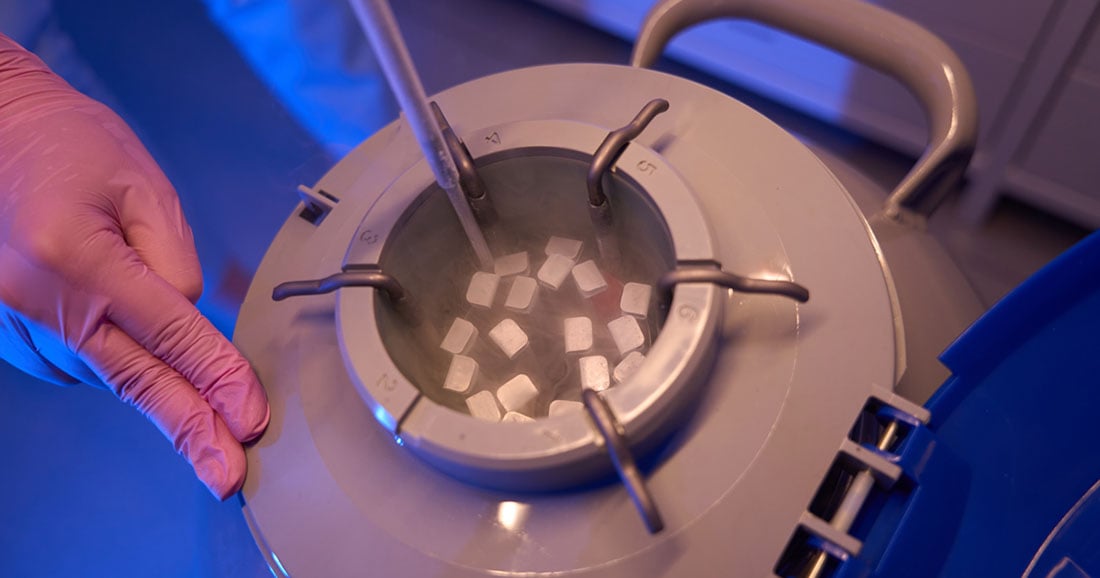
1. Use Validated Packaging and Temperature-Controlled Solutions
Maintaining the cold chain is critical when transporting biological materials. Packaging solutions must be validated to maintain specific temperature ranges throughout transit—whether ambient, refrigerated (2-8°C), frozen (-20°C), or ultra-low temperatures (-80°C or lower).
- Passive packaging (e.g., gel packs, phase change material (PCM) panels, or dry ice) is common for shorter or well-controlled shipments.
- Active packaging (powered temperature-controlled units) offers temperature control without reliance on gel packs or PCM panels, and is suited to shipments containing a larger volume of samples with high temperature-sensitivity on more complex routes, but may not be suitable for all types of biological samples.
Selecting the right packaging depends on sample type, transit time, volume of samples and environmental exposure risk.
2. Ensure Comprehensive Temperature Monitoring
Real-time temperature monitoring is a game-changer for biological sample transport. Using IoT-enabled data loggers or smart sensors that provide continuous temperature tracking ensures immediate visibility into any potential excursions.
Alerts triggered by temperature deviations allow logistics teams to take corrective actions promptly, reducing the risk of compromised samples. Additionally, detailed temperature reports support audit trails and regulatory compliance.
3. Adhere to Regulatory and Biosafety Guidelines
Each shipment must comply with applicable regulations, which may vary by region and sample type. This includes documentation such as:
- Material Safety Data Sheets (MSDS)
- Import/export permits and licences
- Chain of custody records
- Proper labeling (e.g., UN3373 for biological substances) and documentation (e.g., UN2814 / UN2900 infectious substances)
Engaging regulatory experts early in the planning process helps avoid customs delays and legal issues, ensuring seamless cross-border movement.
4. Implement Secure Chain of Custody Procedures
Security is paramount when transporting human tissues and other sensitive biological samples. Chain of custody protocols should be strictly enforced, documenting every handoff—from collection to delivery.
Using tamper-evident seals, secure packaging, and trusted couriers reduces risks of loss, theft, or contamination. Digital tracking systems enhance transparency and accountability at every stage.
5. Train Personnel on Handling and Emergency Protocols
Even the best logistics setup can fail without well-trained personnel. Staff involved in packaging, shipping, and receiving biological samples must understand handling requirements, temperature sensitivities, and emergency procedures.
Regular training programs and audits help maintain high standards and reduce human error.
Conclusion
Transporting biological samples securely and compliantly is a complex but essential part of modern life sciences logistics. By implementing validated packaging, continuous temperature monitoring, strict chain of custody, and regulatory compliance, organizations can protect sample integrity and support the success of research and clinical activities.
For organizations seeking trusted partners in this highly specialized field, Biocair offers end-to-end logistics solutions tailored to the unique demands of biological sample transport. With a global network, cutting-edge technology, and expert regulatory knowledge, Biocair ensures your samples reach their destination safely, on time, and in compliance with regulatory requirements.
Related Contents:

Biocair
Cold Chain Solutions for Life Sciences and Pharma
Biocair is a global logistics provider specializing in pharmaceutical, biotechnology and life sciences supply chain solutions with nearly 40 years of experience. By assembling a team of best-in-class industry experts in quality, cold chain and regulatory compliance, Biocair focuses on providing the most comprehensive time-sensitive and temperature-controlled solutions.