The rapid advancement of cell and gene therapies (CGT) is transforming the treatment landscape for complex diseases like cancer, rare genetic disorders, and autoimmune conditions. These innovative therapies offer personalized, often one-time treatments that can be life-changing — or even life-saving. However, the biological materials used in CGT are highly sensitive, requiring strict environmental control throughout the supply chain.
At the heart of this process is the cryogenic supply chain, a specialized segment of life science logistics that demands ultra-low temperature conditions and flawless execution. As the demand for CGT therapies increases globally, so does the need for robust, validated, and agile cryogenic transportation solutions.
Why Cryogenic Logistics Matters in CGT
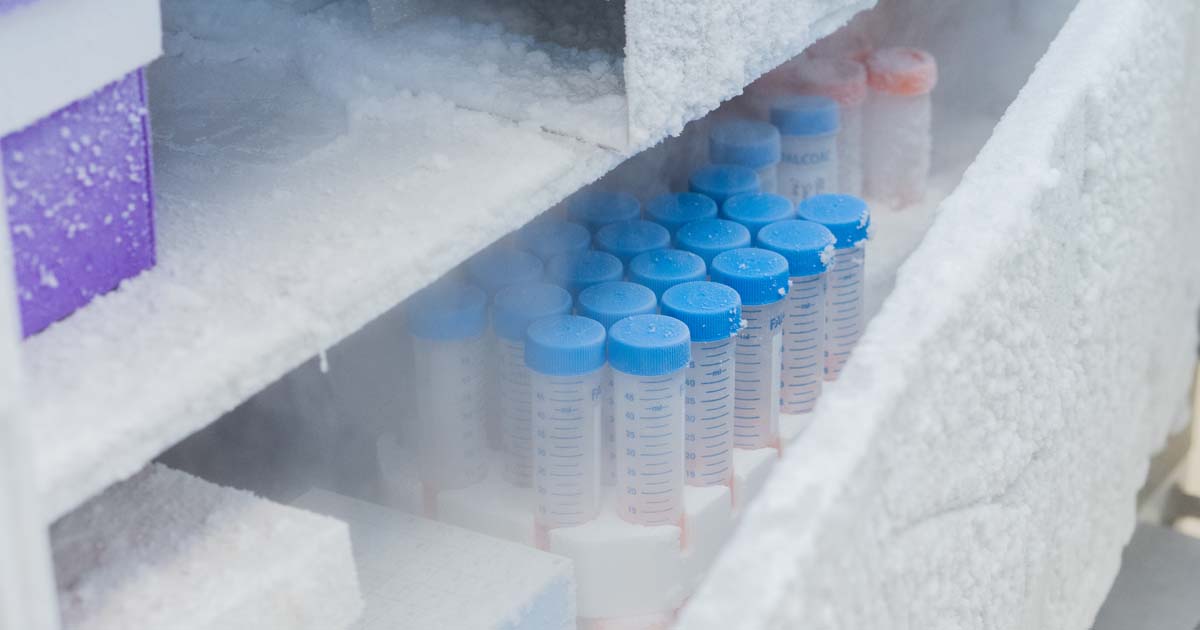
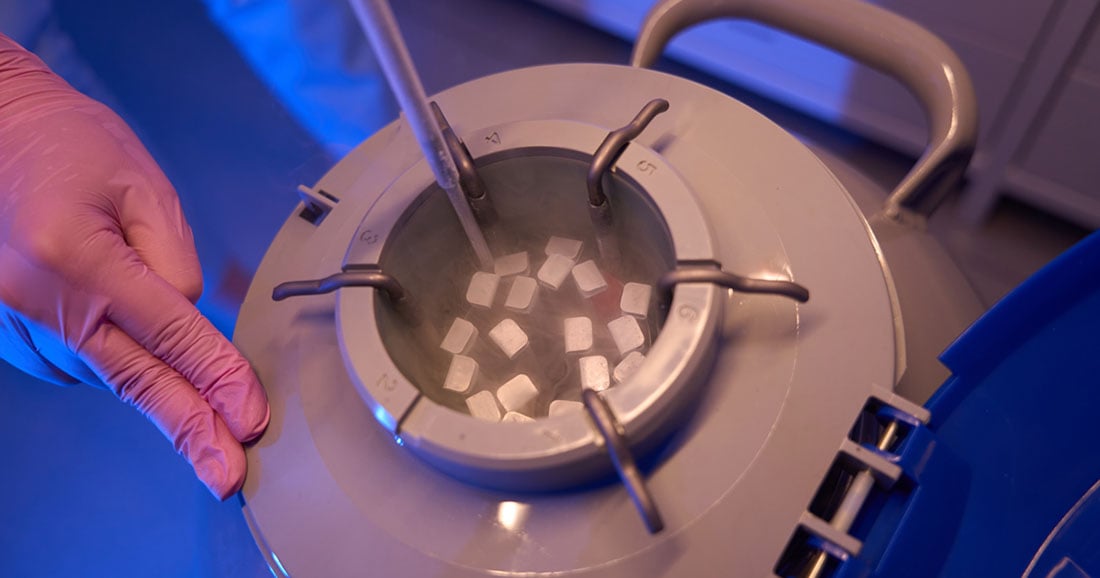
Unlike traditional pharmaceuticals, cell and gene therapies are made using living cells, which must be preserved in extremely cold environments to maintain their viability. Many CGT products require storage and transit at -150°C or below, typically achieved using liquid nitrogen (LN2) vapor-phase containers. This is a far cry from the refrigerated or frozen conditions used in standard cold chain logistics.
Maintaining these conditions across global shipping routes — including customs clearance, airport transfers, and final-mile delivery — requires highly specialized infrastructure and planning. Biocair’s expertise in cell and gene therapy logistics ensures that these challenges are addressed proactively, keeping product integrity and patient safety as top priorities.
Infrastructure and Equipment for Cryogenic Transportation
Cryogenic shipping involves more than just sub-zero storage; it requires purpose-built packaging and validated handling procedures. Biocair utilizes industry-leading cold chain solutions, including:
- Liquid nitrogen dry shippers with 10+ day hold times
- Real-time GPS and condition monitoring (temperature, orientation, shock, and light exposure)
- Pre-qualified, reusable cryogenic containers from globally recognized manufacturers
- End-to-end tracking systems that support chain-of-identity and chain-of-custody compliance
These systems are supported by Biocair’s dedicated lab logistics team, which works closely with researchers, clinical sites, and manufacturing partners to ensure shipments are prepared, packed, and handled according to stringent CGT protocols.
Risk Management in the Cryogenic Supply Chain
With patient-specific therapies, there is no margin for error. A delay, a minor temperature deviation, or mishandling can lead to treatment failure or the need to restart a costly and time-consuming process. That’s why pharma logistics for CGT must include advanced risk mitigation strategies.
Biocair takes a proactive approach to risk management by:
- Conducting route validation and simulation testing
- Monitoring weather and geopolitical risks that may impact shipment timelines
- Offering clinical trial logistics support with full audit trails and regulatory compliance
- Training personnel on CGT-specific handling and emergency response protocols
This holistic strategy enables our team to prevent potential issues before they arise, ensuring each shipment arrives on-time, in-full (OTIF) and in optimal condition.
Supporting a Scalable Cell and Gene Supply Chain
As CGT moves from early-stage research to commercial-scale therapies, logistics solutions must evolve to support higher volumes and global distribution. Biocair’s scalable infrastructure and global network make it possible to support commercial rollout while maintaining the individualized care CGT demands.
From coordinating clinical trial logistics to managing global product launches, our experts deliver tailored, agile services that bridge science and logistics seamlessly.
Final Thoughts
The success of cell and gene therapy logistics hinges on the performance of the cryogenic supply chain. With therapies that depend on ultra-low temperatures, precision timing, and strict regulatory compliance, logistics is more than a support function — it’s a critical component of therapy delivery.
Biocair is proud to lead the way in cryogenic and cold chain logistics for the life sciences, offering fully integrated solutions that prioritize safety, compliance, and speed. Whether you're moving a single patient’s cells across borders or scaling a global CGT program, we ensure your supply chain is ready for the demands of tomorrow’s medicine.
Related Contents:

Biocair
Cold Chain Solutions for Life Sciences and Pharma
Biocair is a global logistics provider specializing in pharmaceutical, biotechnology and life sciences supply chain solutions with nearly 40 years of experience. By assembling a team of best-in-class industry experts in quality, cold chain and regulatory compliance, Biocair focuses on providing the most comprehensive time-sensitive and temperature-controlled solutions.