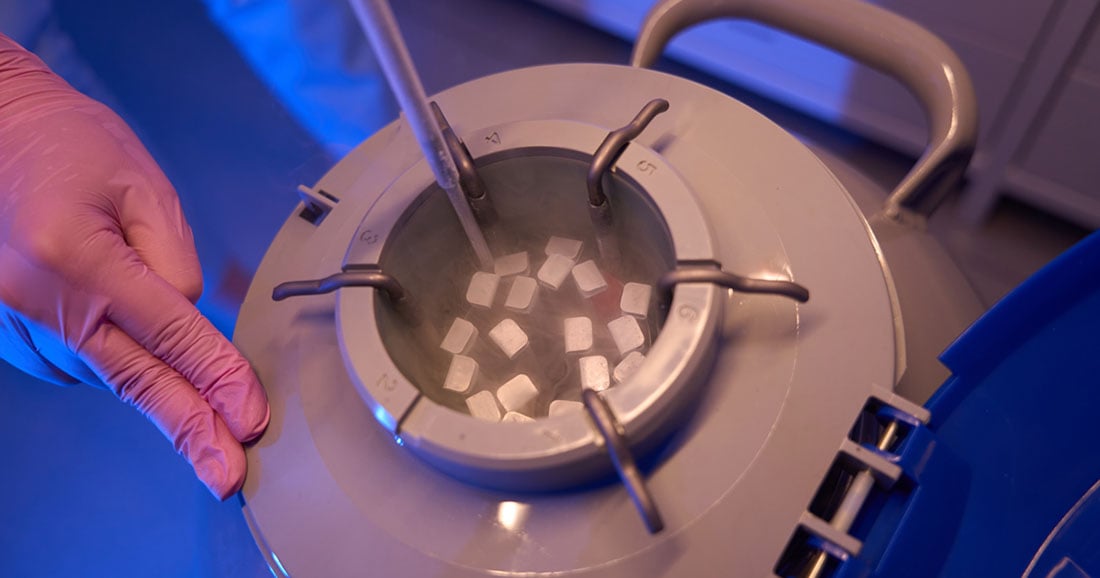
Cell and gene therapies are revolutionizing medicine, offering treatments for previously incurable diseases. However, these therapies present unique logistical challenges, particularly in maintaining their integrity. Temperature control during transportation and storage is crucial to preserving the efficacy of these sensitive products. In this blog, we’ll explore how temperature control in cell and gene logistics plays a vital role in ensuring these therapies reach patients in optimal condition.
Understanding Cell and Gene Therapy Logistics
Cell and gene therapies are highly sensitive to environmental changes, particularly temperature fluctuations. These therapies often involve living cells or genetic material that must be stored and transported under very specific conditions to preserve their potency and effectiveness. Cell and gene logistics is a specialized area within healthcare logistics that focuses on managing the transportation, storage, and distribution of these sensitive products, ensuring they remain viable and safe throughout their journey.
Why Temperature Control is Vital in Cell and Gene Therapy Logistics
- Preserving Biological Integrity:
Cell and gene therapies involve live cells or modified materials that are sensitive to temperature changes. Exposure to improper temperatures can lead to cell damage or reduced efficacy. Temperature-controlled logistics ensure these therapies remain viable, preserving their integrity, whether through cryogenic storage for gene therapies or controlled room temperature for cell-based treatments.
- Minimizing Risk of Product Degradation:
Even small temperature fluctuations during storage or transport can result in the degradation of critical biological components in cell and gene therapies. Cell and gene therapy logistics must incorporate state-of-the-art temperature monitoring systems, such as RFID tags, 5G and real-time data logging, to continuously track temperature conditions throughout the supply chain. If there are any deviations from the desired temperature range, immediate corrective actions can be taken to prevent irreversible damage to the product.
- Regulatory Compliance:
The transportation and distribution of cell and gene therapies are governed by strict regulations to ensure product safety and efficacy. Regulatory bodies such as the FDA, EMA, and other global organizations require meticulous documentation and monitoring of temperature conditions during the entire supply chain process. Temperature control in cell and gene logistics is not just a matter of product quality; it is also a regulatory requirement. Failure to comply with these standards could result in product loss, legal repercussions, and potentially harm patients. Ensuring consistent temperature control and proper monitoring aligns logistics practices with regulatory requirements, mitigating these risks.
- Ensuring Timely Delivery for Treatment Efficacy:
Timeliness is crucial in cell and gene therapies, especially in life-threatening conditions where delays in treatment could have serious consequences. Efficient temperature-controlled logistics help ensure that therapies are delivered on time, with no compromises to the temperature conditions. This enables healthcare providers to administer treatments without delay, improving patient outcomes and ensuring that the therapies have the highest possible chance of success.
Technologies Advancing Temperature Control in Cell and Gene Therapy Logistics
- Real-Time Temperature Monitoring:
Advancements in technology have made it possible to continuously monitor temperature conditions during transportation, allowing logistics teams to make immediate adjustments if necessary. This technology ensures that cell and gene therapies are consistently maintained within the required temperature range throughout the supply chain.
- Advanced Packaging Solutions:
Packaging plays a significant role in temperature control, particularly when it comes to products that require cryogenic storage or precise temperature conditions. Advanced packaging solutions, such as insulated containers and passive or active cooling systems, are used to protect cell and gene therapies from external temperature fluctuations during transit. These packaging solutions are designed to maintain a stable environment for the therapies, even in unpredictable conditions.
- Automated Data Logging Systems:
Automated data logging systems are integral to temperature-controlled logistics. These systems record and document temperature conditions throughout the supply chain, providing a detailed log of any potential deviations. This data not only helps with regulatory compliance but also serves as a safeguard, allowing companies to track and respond to any issues quickly.
Conclusion
Temperature control is crucial for the success of cell and gene therapy logistics. It ensures the integrity of therapies, maintains regulatory compliance, and minimizes degradation risks. As these treatments evolve, investing in advanced logistics solutions will be key to delivering therapies in optimal condition, improving patient outcomes, and transforming lives.
Key Takeaways:
- Temperature control in cell and gene therapy logistics is vital to preserve the biological integrity and efficacy of therapies.
- Real-time temperature monitoring and advanced packaging solutions help ensure products remain viable during transport.
- Regulatory compliance requires stringent temperature control throughout the entire logistics chain.
- Specialist logistics practices ensure timely delivery, which is crucial for the success of life-saving therapies.
Related Content:

Biocair
Cold Chain Solutions for Life Sciences and Pharma
Biocair is a global logistics provider specializing in pharmaceutical, biotechnology and life sciences supply chain solutions with nearly 40 years of experience. By assembling a team of best-in-class industry experts in quality, cold chain and regulatory compliance, Biocair focuses on providing the most comprehensive time-sensitive and temperature-controlled solutions.