When we think of life sciences, we envision innovation, healing, and the future of human health. But behind every vaccine, diagnostic kit, or biologic therapy lies a complex and often high-risk supply chain. Among the most critical — and frequently overlooked — challenges in this landscape is the transportation of dangerous goods.
In life science logistics, safety, compliance, and precision are not optional — they’re the foundation of every shipment.
The Hidden Complexity of Dangerous Goods Transport
In the context of life sciences, “dangerous goods” include more than just hazardous chemicals. They encompass a wide range of sensitive and regulated materials such as:
- Biological substances (Category A and B)
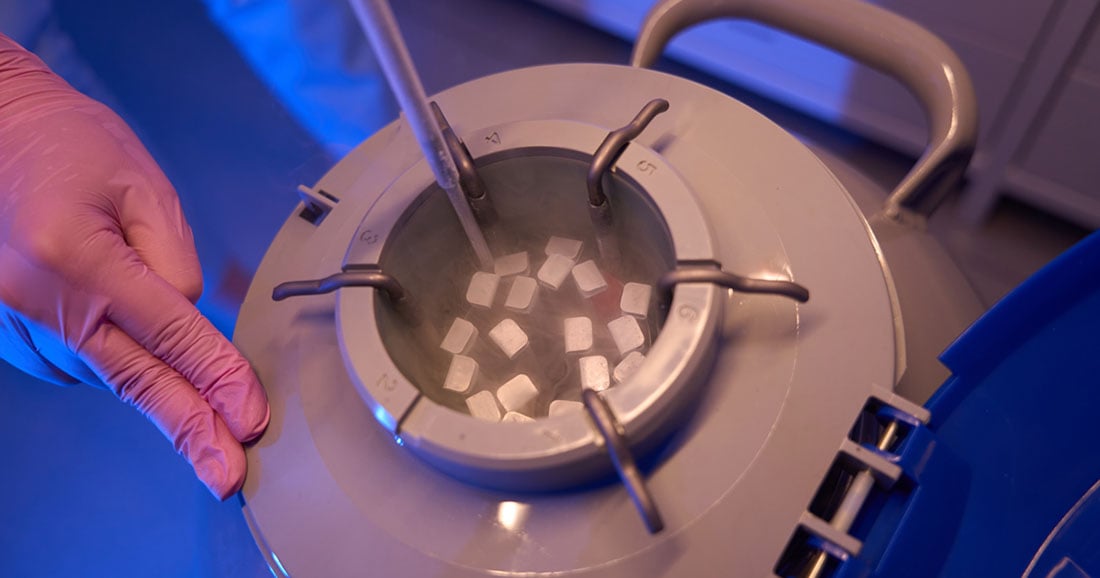
- Dry ice used in cold chain logistics
- Lithium batteries in portable medical devices
- Radioactive tracers for diagnostics and imaging
- Infectious or toxic specimens used in clinical trial supply chains
These aren’t ordinary shipments. They require meticulous handling, real-time monitoring, and expert planning to ensure safety and compliance across borders.
1. Regulatory Navigation in Global Cold Chain Logistics
Each country enforces its own version of dangerous goods transport regulations, including:
- IATA Dangerous Goods Regulations (DGR)
- UN Model Regulations
- ADR and IMDG codes for road and sea transport
- Country-specific customs, health, and safety protocols
Failure to comply with these standards — even in a single documentation detail — can result in costly delays, rejected cargo, or health risks.
2. Precision Packaging for Clinical Trial Supply Chain Success
A significant portion of life science logistics revolves around clinical trials. These supply chains involve shipping time- and temperature-sensitive products like:
- Investigational Medicinal Products (IMPs)
- Biological samples from patients
- Reagents and testing kits
Many of these fall under dangerous goods transport categories and must be packaged with cold chain solutions that conform to regulatory packing specifications. Improper handling of dry ice, for instance, can result in dangerous pressure buildup. Similarly, samples from infectious patients must be triple-packaged, clearly labeled, and secured against leaks.
3. Trained Experts: The Backbone of Safe Logistics
Whether handling cold chain logistics or radioactive materials, well-trained personnel are critical. This includes:
- Certified DG (dangerous goods) specialists
- Cold chain handlers
- Drivers trained in regulatory documentation and emergency protocols
One misstep — a mislabeled box or a missed expiration on a thermal indicator — can jeopardize not only the shipment but the entire clinical trial supply chain.
4. Planning for the Unpredictable
Delays, rerouted flights, customs holds — these are daily risks in cold chain logistics. For dangerous goods transport, contingency planning must go further: emergency response procedures, temperature excursion mitigation, and shipment monitoring are essential safeguards.
The stakes are especially high for clinical research. A compromised vaccine batch or missed patient sample can invalidate weeks of trial work or regulatory submissions.
5. Responsible Logistics for a Safer Tomorrow
Sustainability and compliance must go hand-in-hand. As regulators push for more sustainable logistics, life science companies face the challenge of maintaining cold chain integrity while reducing environmental impact. This includes:
- Using recyclable and reusable packaging
- Optimizing shipping routes to lower emissions
- Exploring carbon-neutral options for life science logistics
Conclusion: Trust, Not Just Transport
In life science logistics, dangerous doesn’t mean reckless. It means regulated, expertly handled, and critically important. Whether moving infectious samples for diagnostics, biologics for therapy, or investigational drugs for clinical trials, every shipment requires trust, precision, and a partner who understands the stakes.
At Biocair, we specialize in the safe, compliant movement of regulated materials — from dangerous goods transport to cold chain logistics — supporting the entire clinical trial supply chain. Because in our world, logistics is more than movement — it's a commitment to helping patients in need.
Related Contents:

Biocair
Cold Chain Solutions for Life Sciences and Pharma
Biocair is a global logistics provider specializing in pharmaceutical, biotechnology and life sciences supply chain solutions with nearly 40 years of experience. By assembling a team of best-in-class industry experts in quality, cold chain and regulatory compliance, Biocair focuses on providing the most comprehensive time-sensitive and temperature-controlled solutions.